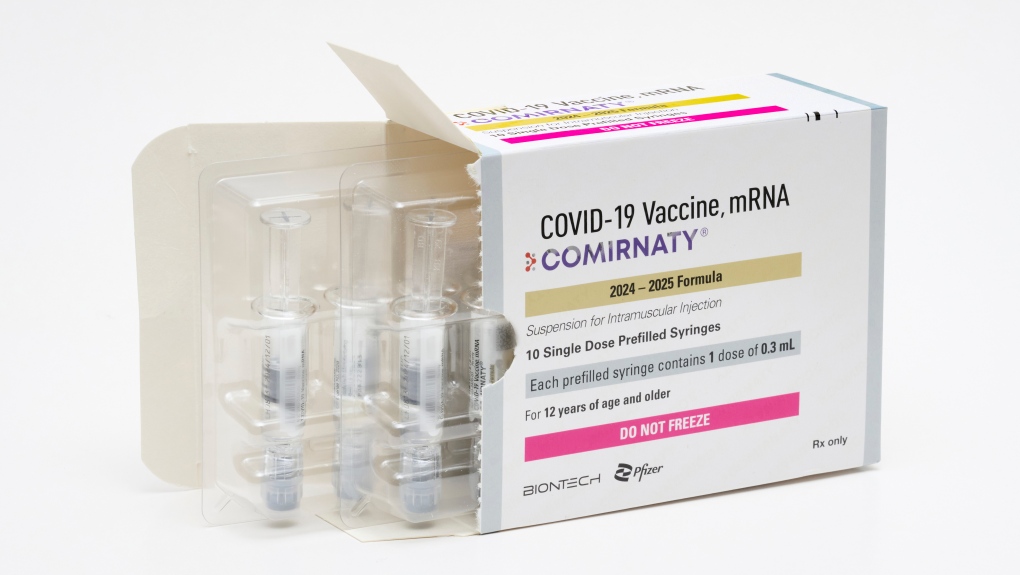
Health Canada approved Pfizer-BioNTech’s updated COVID-19 vaccine on Tuesday, marking its third authorization of vaccine formulations that protect against the most recently circulating variants of the virus.
Pfizer-BioNTech’s mRNA vaccine, called Comirnaty, targets the KP.2 subvariant of Omicron, replacing the previous version that targeted the XBB.1.5 Omicron subvariant.
The approval of Comirnaty follows last week’s authorization of Moderna’s updated Spikevax mRNA vaccine and Novavax’s updated protein-based vaccine, Nuvaxovid.
You must log in or # to comment.